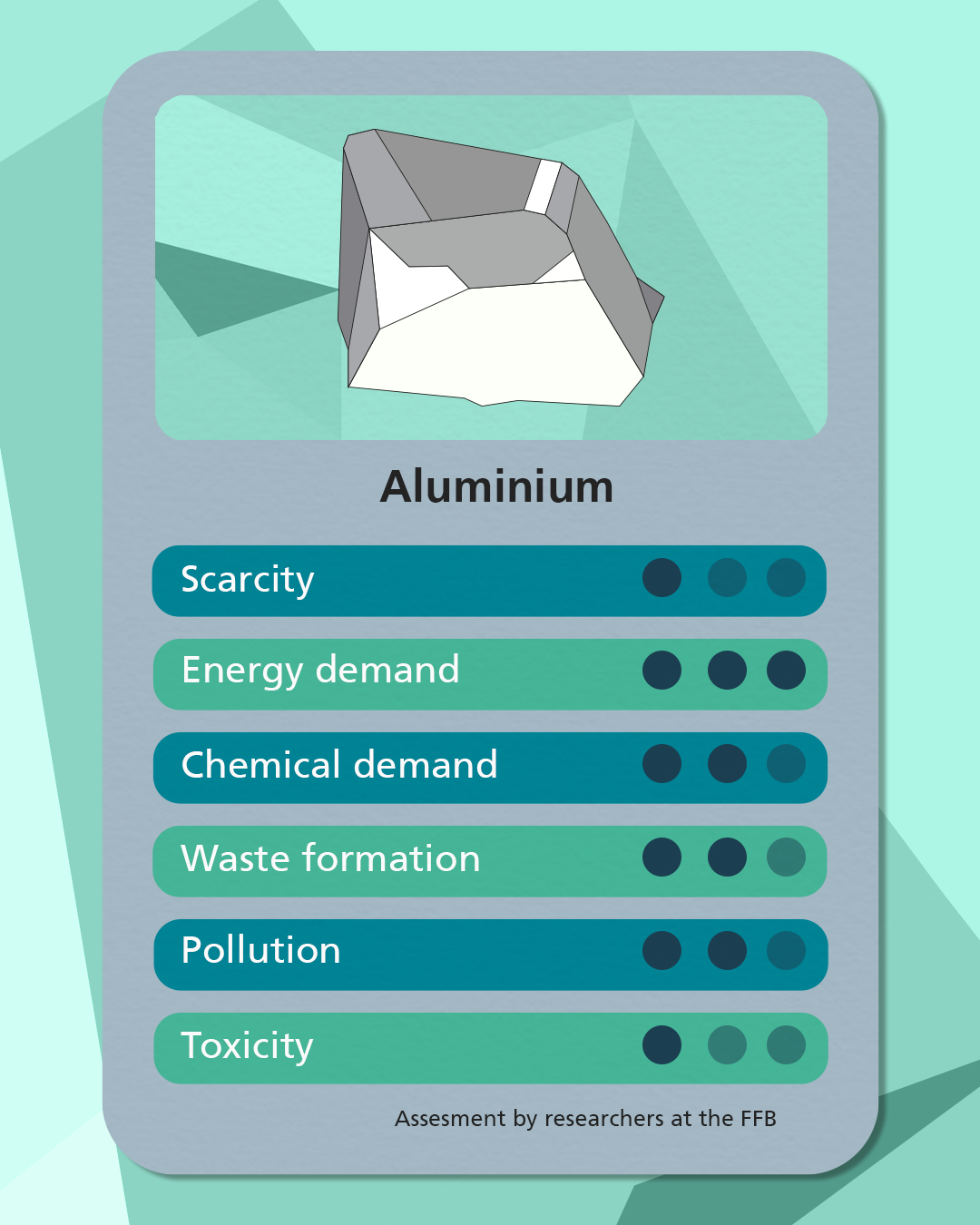
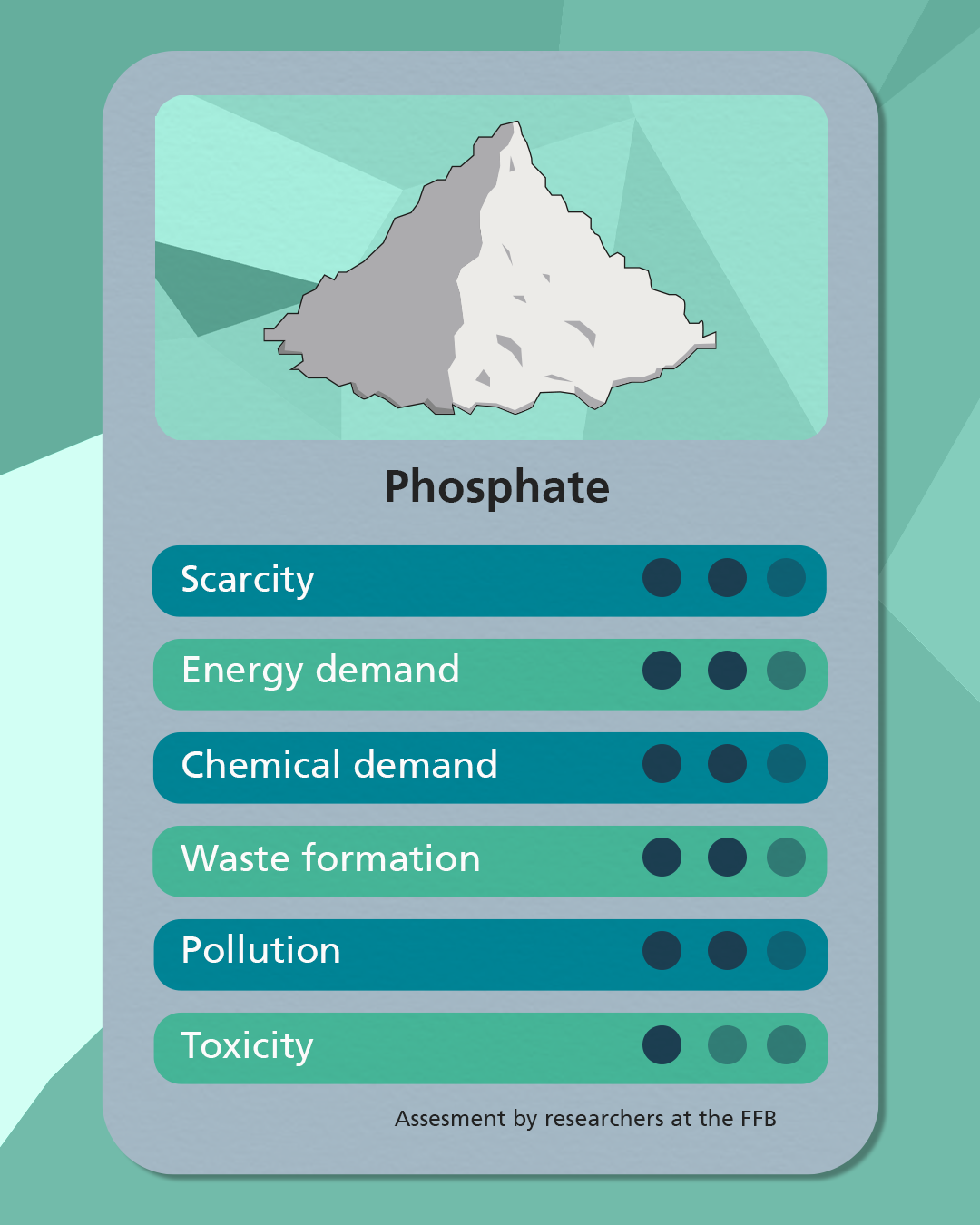
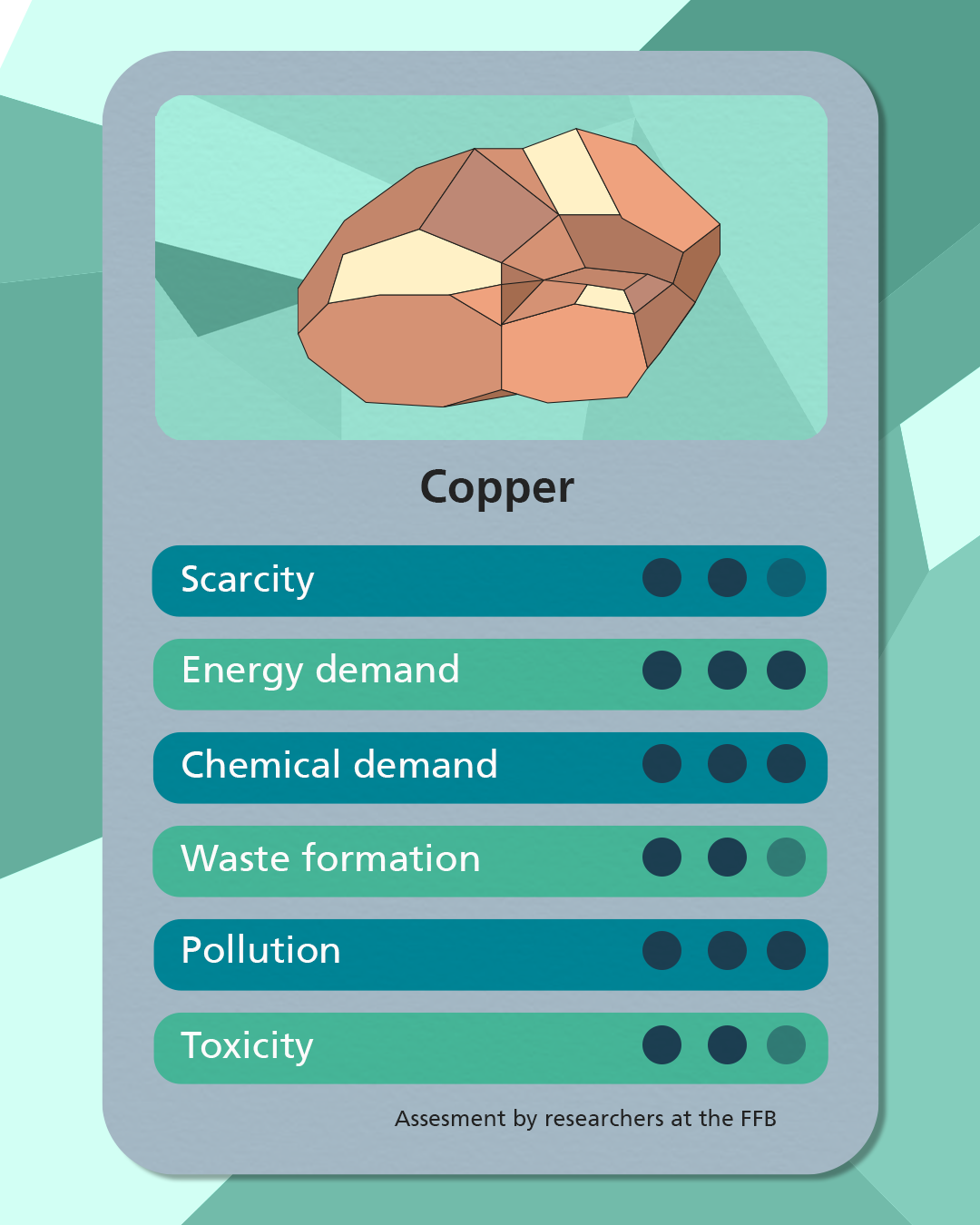
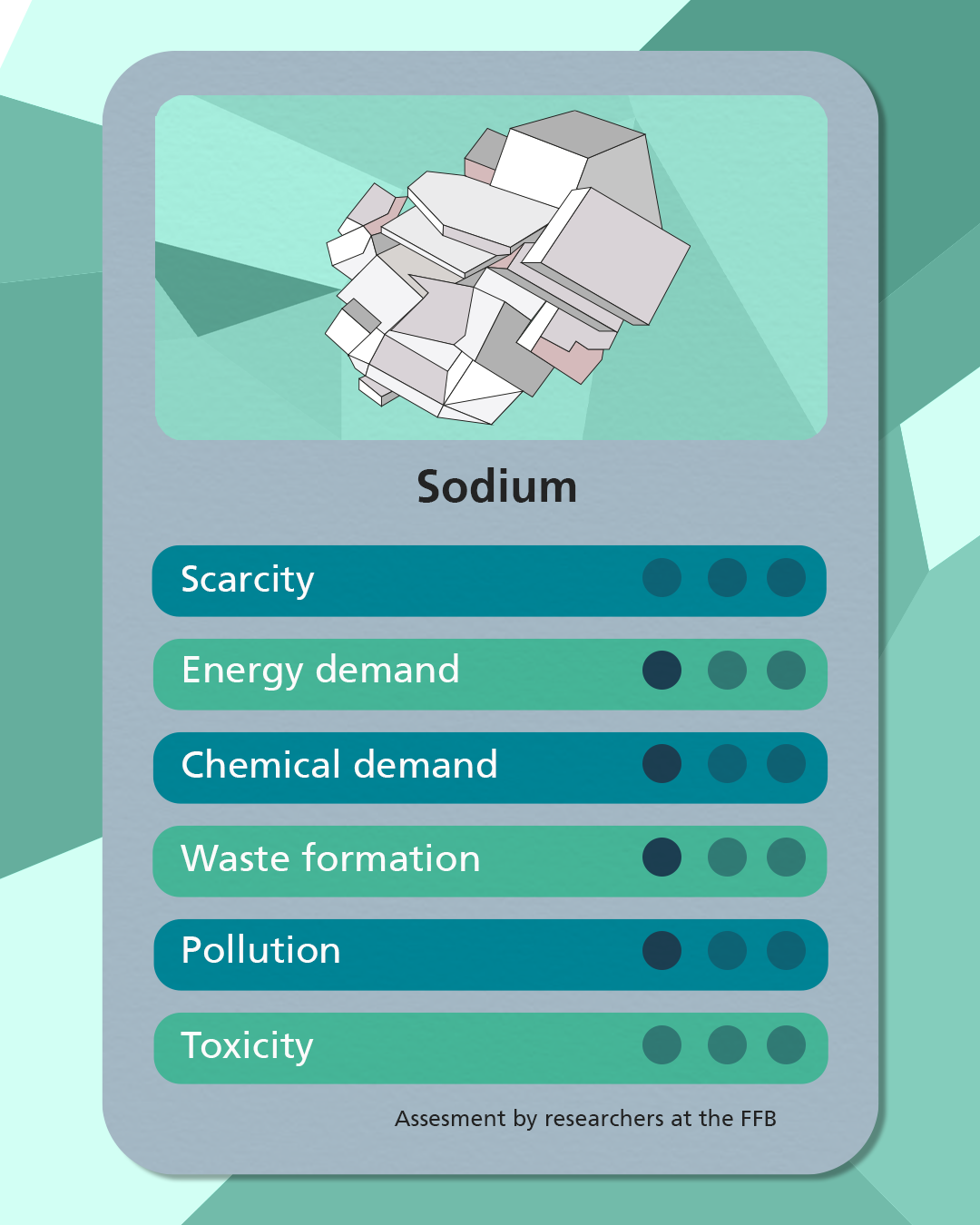
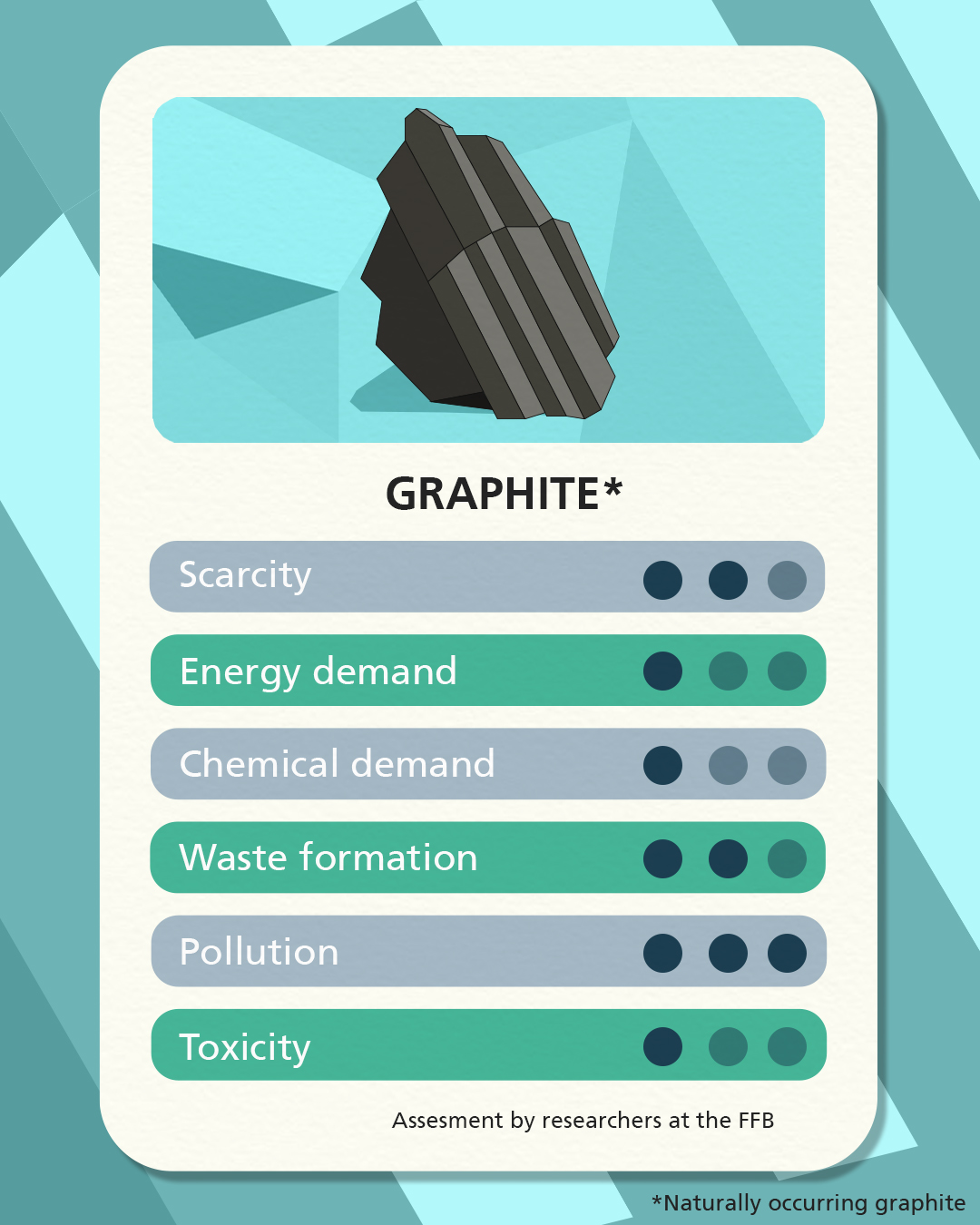
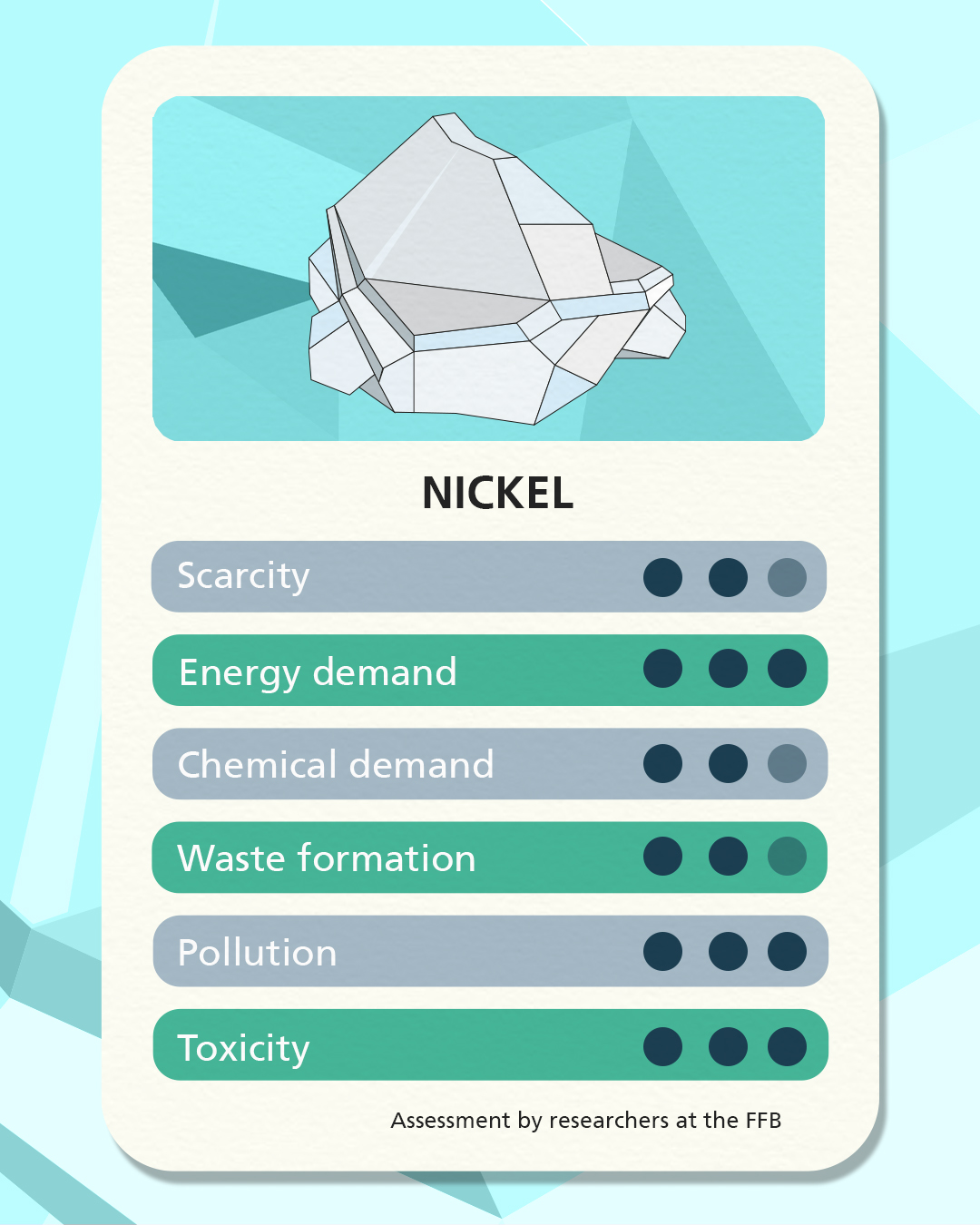
Sodium
The development of sodium-ion batteries (SIBs) as a promising alternative to the widely used lithium-ion batteries (LIBs) is bringing the raw material sodium increasingly into the focus of battery cell production. In contrast to lithium, sodium is not used as a metal, but as an ion, and is available in its natural form. With almost inexhaustible deposits in the earth's continental crust and in seawater, the use of sodium instead of lithium enables a secure supply of raw materials. However, chemical substitution under otherwise identical cell chemistries is currently a challenge, which is why SIBs are still under development, while the market is dominated by LIBs.
Despite this challenge, sodium-ion batteries offer some promising advantages. Due to the natural occurrence of sodium ions in various minerals such as sodium chloride, sodium carbonate or sodium nitrate, the extraction of raw materials is comparatively simple and less problematic. These minerals can be extracted either by mining or from seawater, mainly in the form of NaCl. Sodium chloride in particular is an important source of raw materials and is either used directly or converted into other sodium compounds. The metallic portion of sodium, which is extracted using fused-salt electrolysis, only accounts for a small proportion.
Contrary to their promising properties, SIBs have a lower energy density and a higher weight per kilowatt hour than LIBs, which makes their use in electric vehicles more difficult. Nevertheless, the easy, inexpensive, and global availability of sodium chloride, which is also non-toxic, could make it a promising candidate to supplement or even replace lithium ions in battery cells. Further research and development in the field of sodium ion technology will be crucial to strengthen its competitiveness in the market.