What raw materials are needed to manufacture batteries and how can they be recycled?
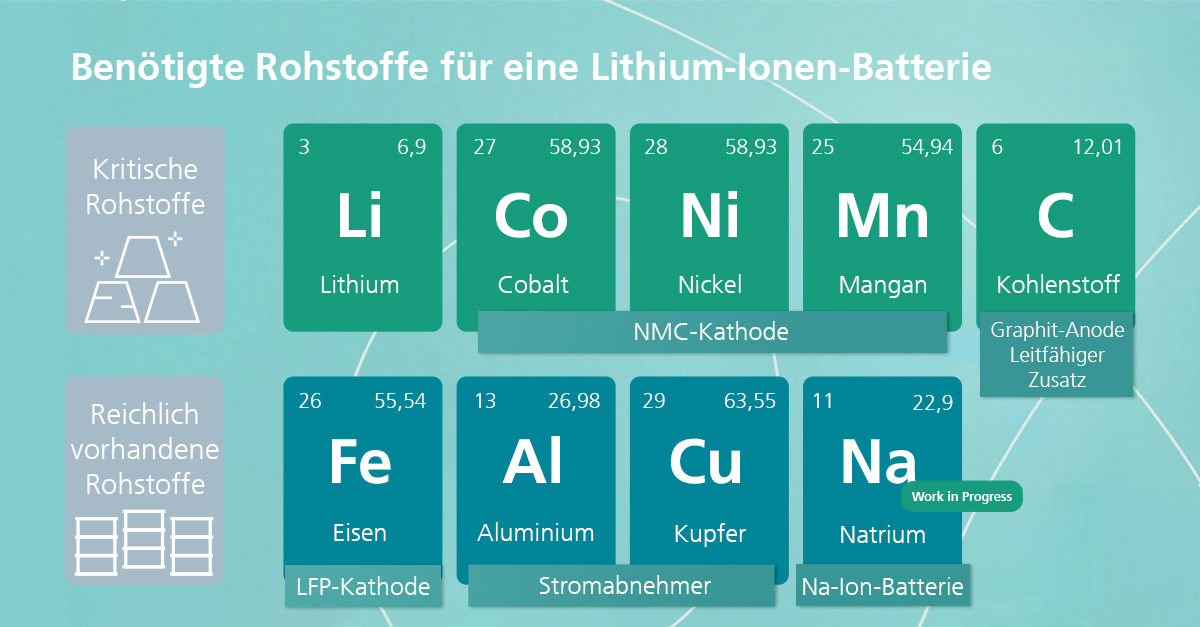
Brief recap: The lithium-ion battery consists of several anode, cathode and separator layers that are soaked in a liquid electrolyte (more on this in second blog post). The performance of the cell, such as its energy density, fast-charging capability or service life (third blog article), is largely determined by their cell materials.
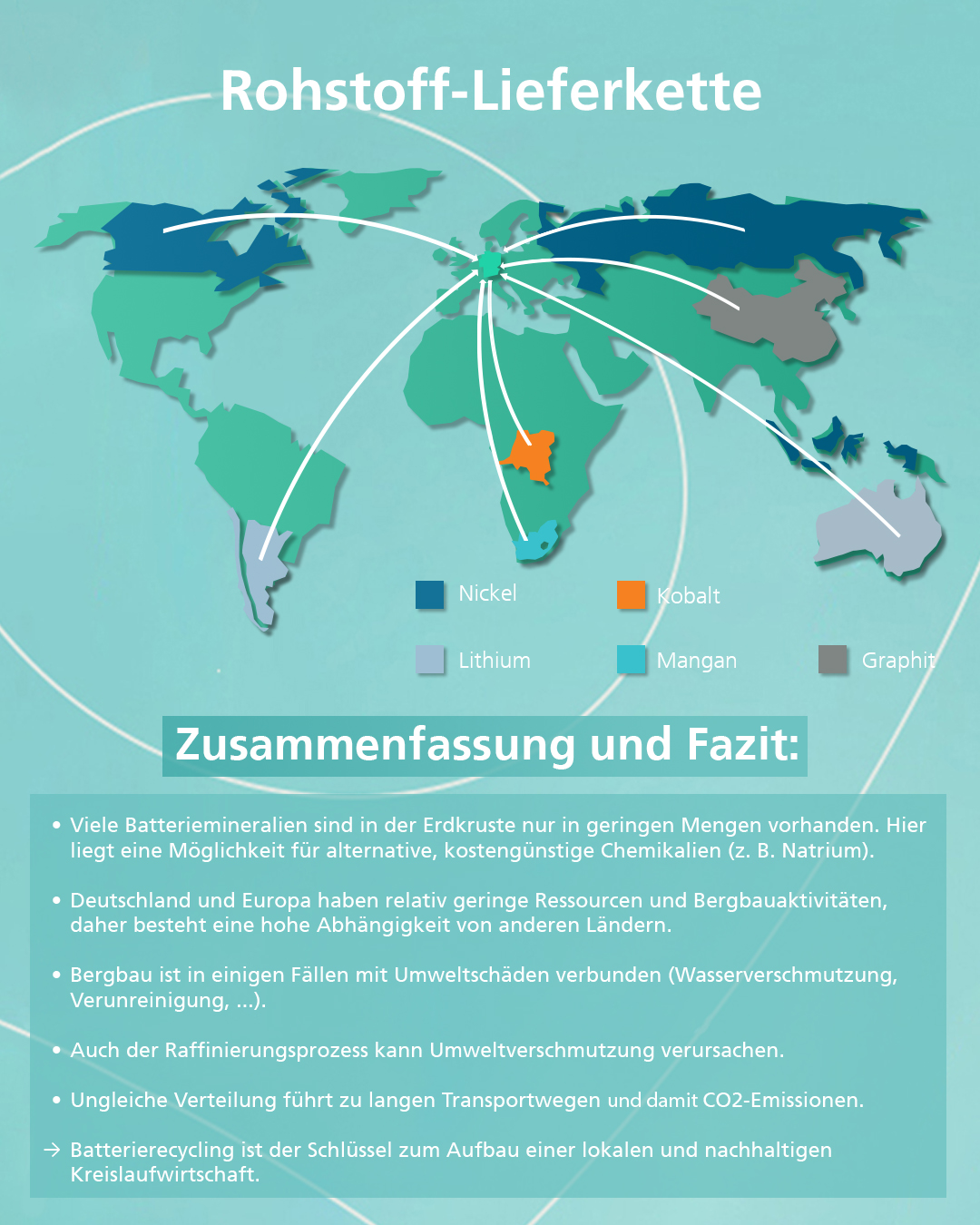
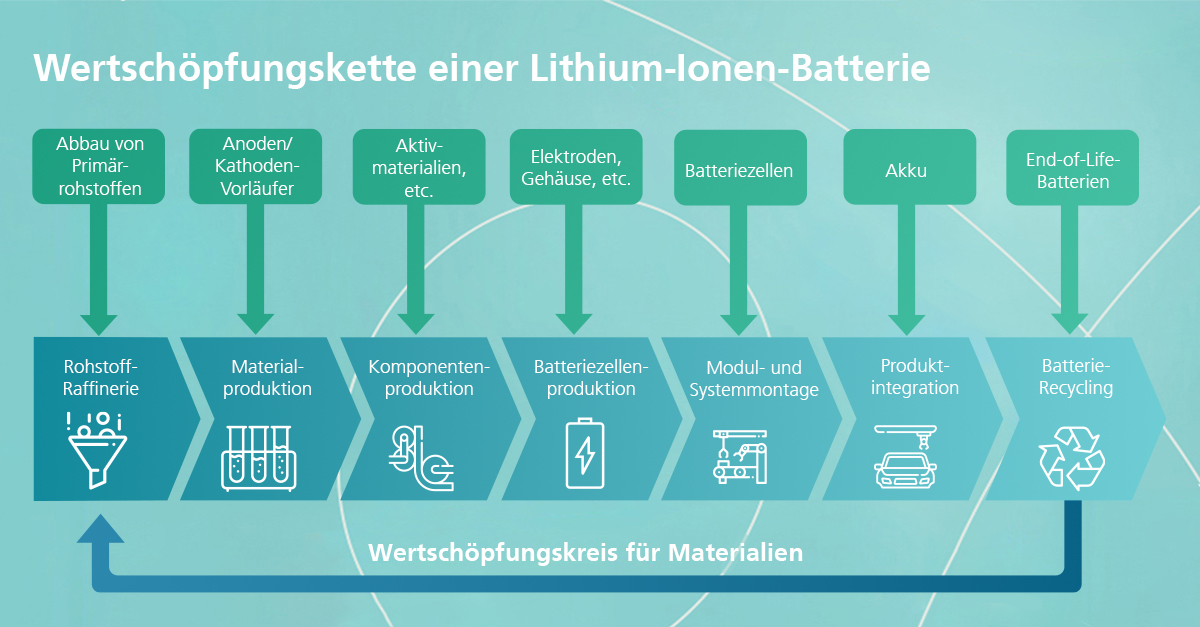
The heart of electromobility, the battery, is unfortunately also its most expensive component, accounting for around fifty percent of total costs (in the case of an electric car). Current prices of battery packs amount to around 137 euros per kilowatt hour.[1] The materials of the battery components, which are a key factor in determining their performance figures, account for a large proportion of the costs. In view of the increasing demand, it is therefore important in the long term to research alternative technologies that use other materials (e.g. cobalt-free cathodes or sodium-based cell chemistries) and recycling options. The seventh blog article in our "Skill and Scale-up" information campaign explains the basics of battery (raw) materials and recycling.