Cell Assembly
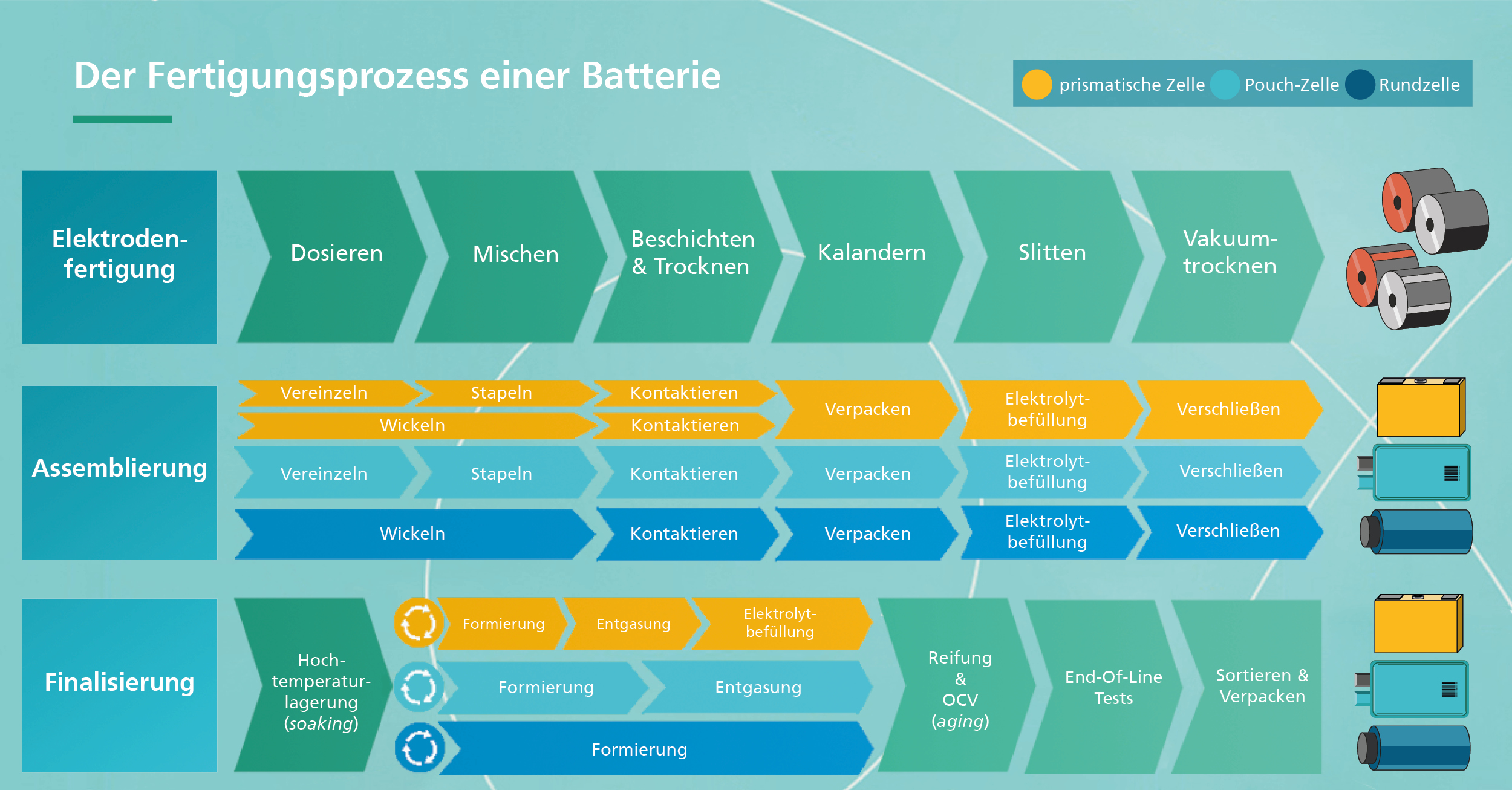
While the electrode production is the same for all cell formats, the assembly process differs depending on the cell type. For this reason, each cell format requires its own assembly line. This process step, however, places high demands on cleanliness and dryness to ensure the smooth, high-quality and safe production of battery cells. For example, the so-called clean and dry rooms must maintain a dry room climate, resulting in very low humidity at a dew point of minus 60 degrees Celsius - the temperature at which moisture begins to condense. This is drier than the desert.
In assembly, the first step is to unwind the dried daughter coils and feed them to the appropriate separation station. Separation means that the individual sheets are then punched out (mechanical separation) or lasered (thermal separation) in order to be stacked into a cell stack or wound into a round cell in the next process step. This means that the anode is followed by the separator, the cathode, and so on. The most common methods to date are single sheet stacking, i.e. the individual electrodes are stacked alternately, or Z-folding. Here, the separator is clamped in the middle and the separated electrode sheets are inserted alternately from the left and right into a separator pocket.
In order to function, each battery cell requires cathode and anode contact tabs. For this purpose, the current tabs of the electrode stack are welded to the contact tabs of the cells during the subsequent "contacting" process. The battery cells must then be packaged. Pouch cells are packaged in an aluminum composite film (pouch film) and sealed gas-tight in three places. One side is left open for electrolyte filling. This is done with a very precise metering lance. Once the cell is filled, it is welded at the last missing point. The cell is now ready to be electrically charged and discharged for the first time.