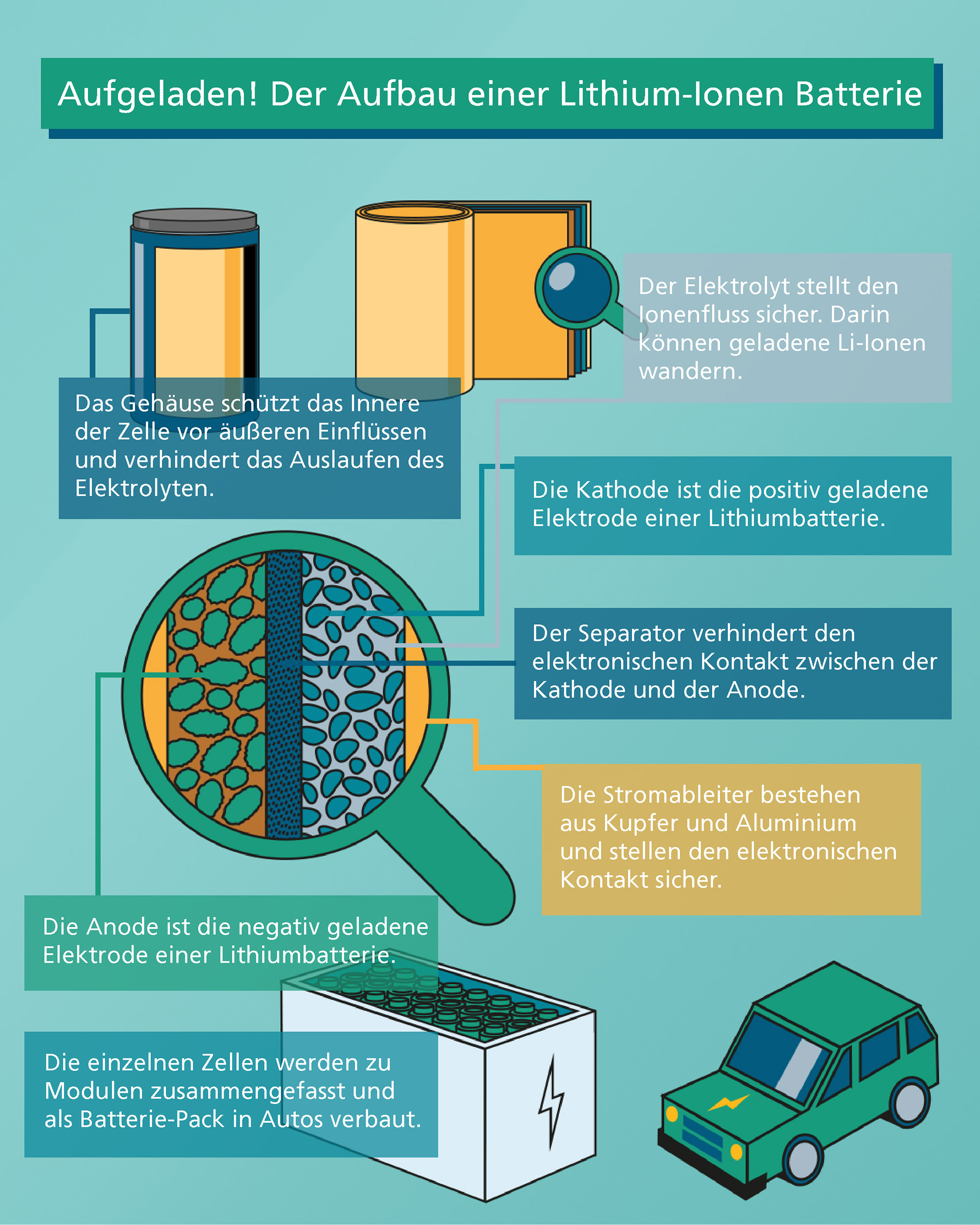
How does a battery work?
They make our world rechargeable. They are light, can store a lot of energy and last a long time. About 30 years ago, pioneers laid the foundation for what is now indispensable in almost all areas of our daily lives: the lithium-ion battery. But how exactly is a battery built and how does it work? We explain this in our second blog post as part of the "SkillandScaleUp" information campaign.
It all started with the realization that lithium loves to make electrodes. The soft material, shiny when freshly cut, is the lightest metal on Earth and very reactive. The world's largest lithium reserves are in Chile and Australia. By 2020, they will account for nearly 75 percent of global mine production, according to DERA (2023). For a long time, however, lithium received little attention. It was not until the research of John Goodenough, Stanley Whittingham and Akira Yoshino, which was based on each other, that lithium received the attention it still receives today.